
Bio199 - Monday 5:30 PM to 8:00 PM
Larval developmental studies in cardiac function, growth and innate behaviors, Room MDS 155B
|
|
 |
|||
|
Bio199 - Monday 5:30 PM to 8:00 PM Larval developmental studies in cardiac function, growth and innate behaviors, Room MDS 155B |
||||
|
|
|||
|
The purpose of this interactive program is engage in authentic scientific discovery related to larval developmental studies in cardiac function, growth and innate behaviors in Drosophila (fruit flies). |
|||
|
Too often life science is distilled into disparate facts addressing major biological concepts, but lacking purpose for learning and applying knowledge to real world contexts. The driving principle of this project is to to foster authentic scientific investigation in biological sciences. |
|||
|
|
|||
|
Resources: Papers and web links and power points 1st day of class ppt : download here
FREE
NEUROPHYSIOLOGY TEXT BOOK ON LINE (use for a review of basic concepts) Free background information on neuron morphology, conducting electrical, ionic bases of resting & action potential, and Synapse structure Participation in conducting analysis of synaptic responses will be the main focus in this course. |
|||
|
Background information for teaching about altering neural activity in these excercises: Regulation in activating inhibitory and excitatory neurons while monitoring effects on acute and chronic behaviors emphasis the importance of these neural circuits. To do this type of manipulation within in an organism which is easy to rear and maintain is ideal for hands on inquiry based learning for courses which emphasize life science topics. This teaching module is designed to integrate modern genetics, engineering, physics, life sciences, modeling and experimental design. Researching the primary scientific literature and the utilizing the related findings as well as postulating the outcome for newly designed experiments based on the results one collects, the students can test their own predictions and draw hypotheses. This approach provides autonomous learning within and among student groups. The measureable outcomes with obtaining quantitative data for analysis and interpretation is a valuable learning experience. Based on one’s findings in the initial experiments one can readily redesign experimental paradigms to test the formulated hypotheses utilizing one’s own prior data. The integration with Arduino hardware and software opens the doors for students to a world of writing code with an experimental purpose and independence in experimental design. The underlying science in these modules focuses on neurobiology. The seminal discoveries by Hubel and Wiesel (1970) demonstrated that activity in sensory input and within the CNS is key in the development and maintenance of neural circuits. This concept is also important for development and maintained synaptic establishment at neuromuscular junction (NMJ) of skeletal muscles (Balice-Gordon et al., 1990; Lomo, 2003). In some cases, the activity profile must occur prior to developmental time points to have plasticity before the neural circuits become more hardwired. After the critical period in synaptic formation, the circuit is not as dependent on activity for competition with other neurons for the establishment of connections. This fundamental phenomenon occurs in organisms from fruit flies to humans. It is known in mice that even after established connections are made as adults that the terminals at NMJs are not fixed to on spot on the muscle fiber. The motor nerve terminals grow out and pull back over time while continuing to communicate with the muscle (Lichtman and Sanes, 2003) If motor neurons which are normally innervating a muscle are removed than other motor neurons will take control of the target and innervate. Thus, motor nerves are searching out targets not all ready committed by other synaptic inputs (Chang and Keshishian, 1996). This was examined in embryonic and larval Drosophila by laser ablating various skeletal muscle fibers during development. Even pharmacological activating or silencing neural circuits during development can have long term consequences in neural connections and overall physiological functions (Smith et al., 2015). For example exposing rodents to nicotine during development changes the dendritic morphology within the CNS which lasts into adulthood (McDonald et al., 2005). Even short exposures in the juvenile stages have long lasting effects in adults for these mice (Ehlinger et al., 2015). Thus, long term consequences in the established neural circuitry within the CNS and at the NMJ can occur based on neural activity when the initial circuits are being formed. A guided self-inquiry based approach to learning science has been demonstrated to being a very effective means for student learning over the long term (Bradforth, et al., 2015; Waldrop 2015). The engineering design with the Arduino systems is a very engaging educational experience sought after in many schools within the USA and abroad. Students can design the experiments with various computer codes to control the duration of light on-off time period and frequency of stimulation to observe how activating or inhibiting specific set on neurons alter development and behavior of the Drosophila larvae or adults. Since many of the experimental paradigms will be novel and many unanswered questions remain to be answered in neurobiology, students may uncover unique findings worthy of publication in scientific journals. Why use flies ? (PDF 1, 2, 3, 4 flies and human disease ) Table of which lines activate which type of neurons (PDF, MS Word ) Recording of an Introduction to the lab: used for Bio 350 (animal physiology, Univ. of KY) dowload (movie here 123 MB) (power point only here) Sample movies of larval behavior: (these are large movies so need to download 1st and then open). Hot linked so click on title. Motor neurons: OK371-ChR2 minus ATR third instar-L1 OK371-ChR2 plus ATR third instar-L1b
Research articles: Abdrakhmanov, M.M., Petrov, A.M., Grigoryev, P.N., Zefirov, A.L.(2013) Depolarization-induced calcium-independent synaptic vesicle exo- and endocytosis at frog motor nerve terminals. Acta Naturae 5(4):77-82. Abstract http://www.ncbi.nlm.nih.gov/pubmed/24455186 Bradacs, H., Cooper, R.L., Msghina, M., and Atwood, H.L. (1997) Differential physiology and morphology of phasic and tonic motor axons in a crayfish limb extensor muscle. Journal of Experimental Biology 200:677-691. [FullText.pdf] Caldwell, L., Harries, P., Sydlik, S. and Schwiening, C.J. (2013) Presynaptic pH and Vesicle Fusion in Drosophila Larvae Neurones.SYNAPSE 67:729–740. [FullText.pdf] Cooper, A.S., and Cooper, R.L. (2009) Historical view and demonstration of physiology at the NMJ at the crayfish opener muscle. Journal of Visualized Experiments (JoVE). JoVE. 33. http://www.jove.com/index/details.stp?id=1595; doi: 10.3791/1595. Text part of video article [PDF]. Cooper, R.L., Harrington, C. Marin, L., and Atwood, H.L. (1996) Quantal release at visualized terminals of crayfish motor axon: Intraterminal and regional differences. Journal of Comparative Neurology 375:583-600 [Abstract] [Full text PDF] Cooper, R.L., Marin, L., and Atwood, H.L. (1995) Synaptic differentiation of a single motor neuron: conjoint definition of transmitter release, presynaptic calcium signals, and ultrastructure. Journal of Neuroscience 15:4209-4222 [Abstract] [pdf] Cooper, R.L., Stewart, B.A., Wojtowicz, J.M., Wang, S., and Atwood, H.L. (1995) Quantal measurement and analysis methods compared for crayfish and Drosophila neuromuscular junctions and rat hippocampus. Journal of Neuroscience Methods 61:67-78 [Abstract] [PDF] Cooper, R.L. and Ruffner, M.E. (1998) Depression of synaptic efficacy at intermolt in crayfish neuromuscular junctions by 20-Hydroxyecdysone, a molting hormone. Journal of Neurophysiology 79:1931-1941 [Abstract] [FullText.pdf] Lancaster, M., Viele, K., Johnstone, A.F.M., and Cooper, R.L. (2007) Automated classification of evoked quantal events. Journal of Neuroscience Methods 159: 325-336. [PDF] Lee, J.-Y., Bhatt, D., Bhatt, D., Chung, W.-Y., and Cooper, R.L. (2009) Furthering pharmacological and physiological assessment of the glutamatergic receptors at the Drosophila neuromuscular junction. Comparative Biochemistry and Physiology, Part C 150: 546–557[PDF]. Li, H. , Peng, X., and Cooper, R.L. (2002) Development of Drosophila larval neuromuscular junctions: Maintaining synaptic strength. Neuroscience 115:505-513 [PDF] Viele, K., Stromberg, A., and Cooper, R.L. (2003) Determining the number of release sites within the nerve terminal by statistical analysis of synaptic current characteristics. Synapse 47:15-25 [PDF] Wu, W.H. and Cooper, R.L. (2010) Physiological recordings of high and low output NMJs on the Crayfish leg extensor muscle. Journal of Visualized Experiments (JoVE). Jove 45: http://www.jove.com/index/details.stp?id=2319 , doi:10.3791/2319 [PDF of paper] Wu, W.-H. and Cooper, R.L. (2012) The regulation and packaging of synaptic vesicles as related to recruitment within glutamatergic synapses. Neuroscience 225:185-198. [PDF] Wu, W.-H. and Cooper, R.L. (2013) Physiological separation of vesicle pools in low- and high-output nerve terminals. Neuroscience Research 75: 275–282. [PDF]
Resources: Growth and development 1. Neat web link about Fruit flies http://www.flyfacility.ls.manchester.ac.uk/forthepublic/ 2. Reproductive potential predicts longevity of female Mediterranvan
fruitflies (PDF)
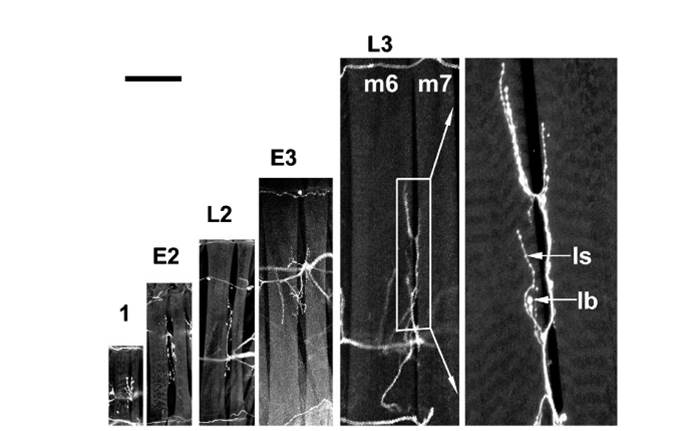
33. Growth and variation of the larvae of Drosophila M Alptov 1929 (PDF) |
|||
|
|
|||
|
website
maintained by Robin L. Cooper. Contact: RLCOOP1 at UKY.EDU |