
NSF funded
RESEARCH EXPERIENCE FOR HIGH SCHOOL SCIENCE TEACHERS

Text: Handouts will be provided
Summer 2005:
Biology Build.
room B08
| Back to Home Page |  | NSF funded RESEARCH EXPERIENCE FOR HIGH SCHOOL SCIENCE TEACHERS |  | ||||
|
| |||||||
|
Text: Handouts will be provided | |||||||
|
Summer 2005: Biology Build. room B08 | |||||||
| | |||||
 | |||||
| Dr.
Robin L. Cooper WWW Home page (go to) | Ms.
Heidi Melcher Anderson www
page: (go to) | Ms.
Barrie Hart www page: (go to) | |||
| | ||
| PHOTOS (go to) | SUMMER 2005 organization (below) | Readings (go to) |
| SUMMER 2005 organization | ||||
|
The specific aims for the 7 weeks of intensive study are:
2.
Learn life history of the model animal "the crayfish" through brief
readings, direct observation and discussion. In addition, learn how crayfish behave
and how behavior is modulated by biogenic amines such as serotonin and the molting
hormone ecdysone. (refs from lab work). (DAY 1) 3.
Learn how neurons communicate with other neurons and muscle. The complete circuit
of sensory-CNS-motor-muscle function is to be understood. Readings in Neuron to
Brain by J.G. Nicholls, 4th ed (I was one of the readers for these chapters during
their construction) and discussion on the subject through didactic intense classroom
instruction are to be implemented during the initial training days. (DAYS 2 and
3) 4.
Dissect crayfish muscle preparations and stain the motor nerves, terminals and
muscle with vital dyes for viewing live preparations. Complete staining procedures
with immunocytochemical techniques that demarcate synaptic regions within the
terminals by light microscopy (i.e., anti-synaptotagmin staining) (Cooper et al.,
1995a, 1996a). (Within WEEK 1). 5.
Learn how to measure synaptic potentials with intracellular recordings in the
crayfish opener muscle preparation. Examine the effects of biogenic amines and
short-term facilitation on the synaptic responses. Learn how to measure and quantify
the synaptic responses. (Crider and Cooper, 2000a) (Within WEEK 1). 6.
Participate in the research of obtaining quantal recordings from nerve terminals
with the focal macropatch technique and learn how to generally analyze the obtained
traces for the 1st pass analysis. (Cooper et al., 1995b) (WEEKS 2-4). 7.
Participate in processing the tissue for electron microscopy that was used for
obtaining the electrophysiogical data in #6 above. (WEEK 2-4). 8.
Participate in electron microscopy of the processed tissue. Learn to thin section,
image and obtain publication quality figures of the synaptic regions of interest.
Participate in processing the images for data analysis of synaptic structure,
vesicle pools and 3D rendering. Mr. Johnstone will help in this training. (Week
5). 9.
Participate in processing the electrophysiological data with stochastic methods
designed by Dr. Kert Viele (Co-PI on active NSF grant in which this supplement
request is affiliated). The teachers will serve as Beta testers of algorithms
that were constructed for automated analysis of sub-quantal measures. A major
Aim of the parent NSF grant was to develop and disseminate software for others
to use related to quantal analysis of synaptic transmission. (Week 6). 10.
Write up the investigations they covered along with completed graphical analysis
of the data and information they obtained. (Week 6). 11.
In exchange for helping with my research project, we would like to spend 1 week
developing novel physiological experiments that can be readily implemented into
high school classes rooms that only posses the basic instruments generally available
to high school science teachers in the US. The learning modules will be made available
through postings on Amer. Physiol. Soc. Educational www pages as well as through
the Digital Library www sites in education currently under construction by SICB
(Soc. for Integrative and Comparative Biology). (Week 7). 12.
To make several contacts within Biology, Chemistry and Statistics depts. on the
Univ. of KY campus in order to build a rapport
with the University faculty. (Weeks 1-7). | ||||
FOR NOW - papers that were distributed May 17, 2005
| ||||
|  |  |  | |
Now how do I cook this critter ? | ||||
 | 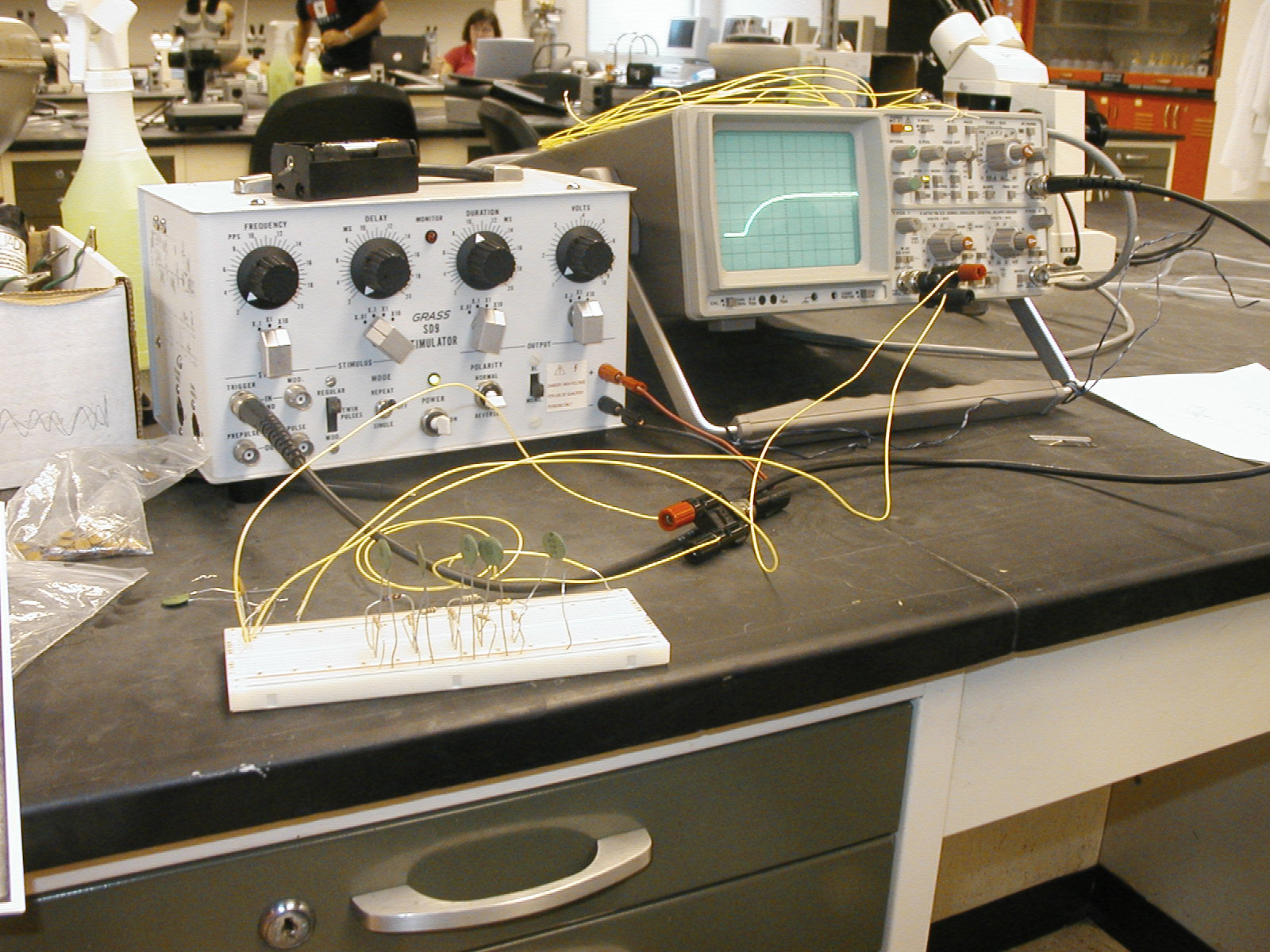 | |||
| The axon coming to life | ||||
 |  | 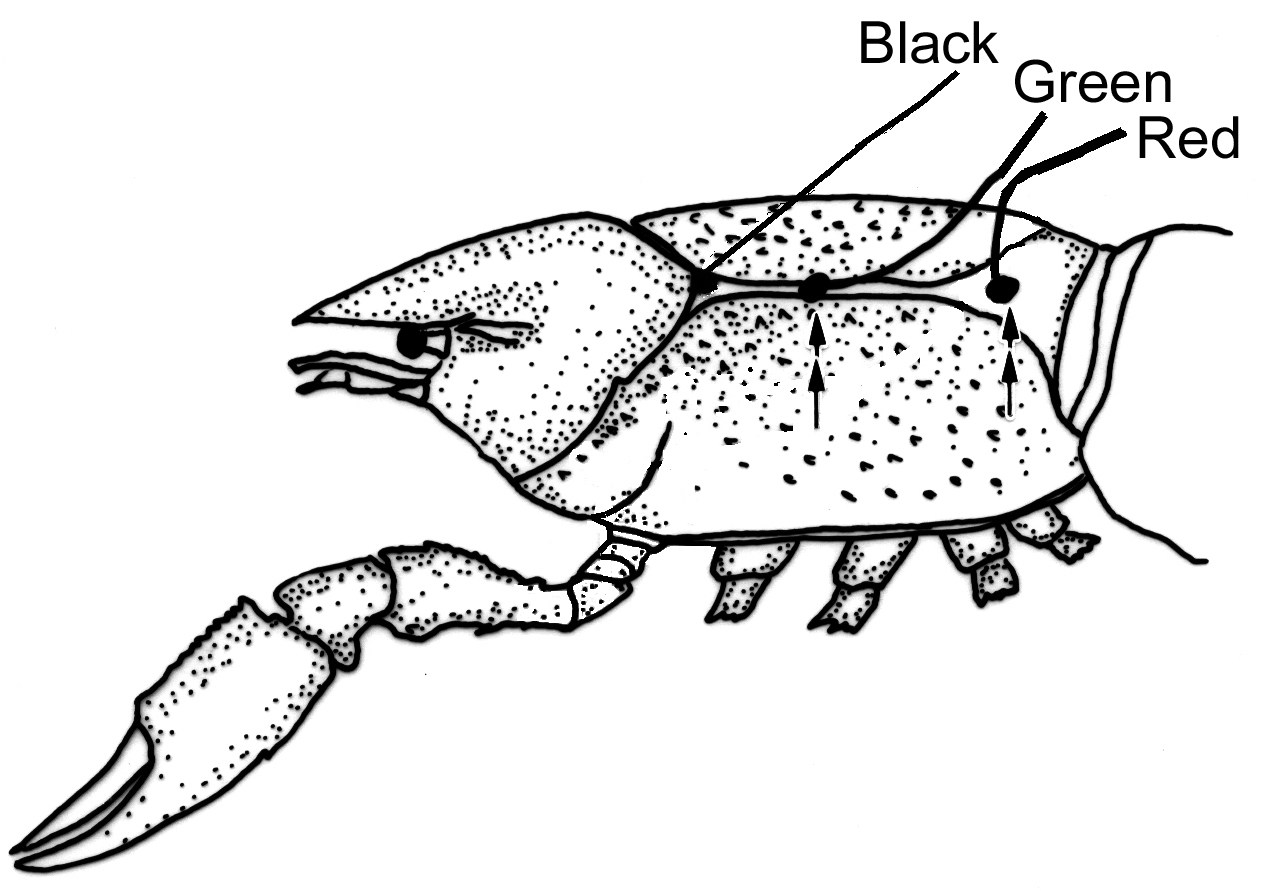 | 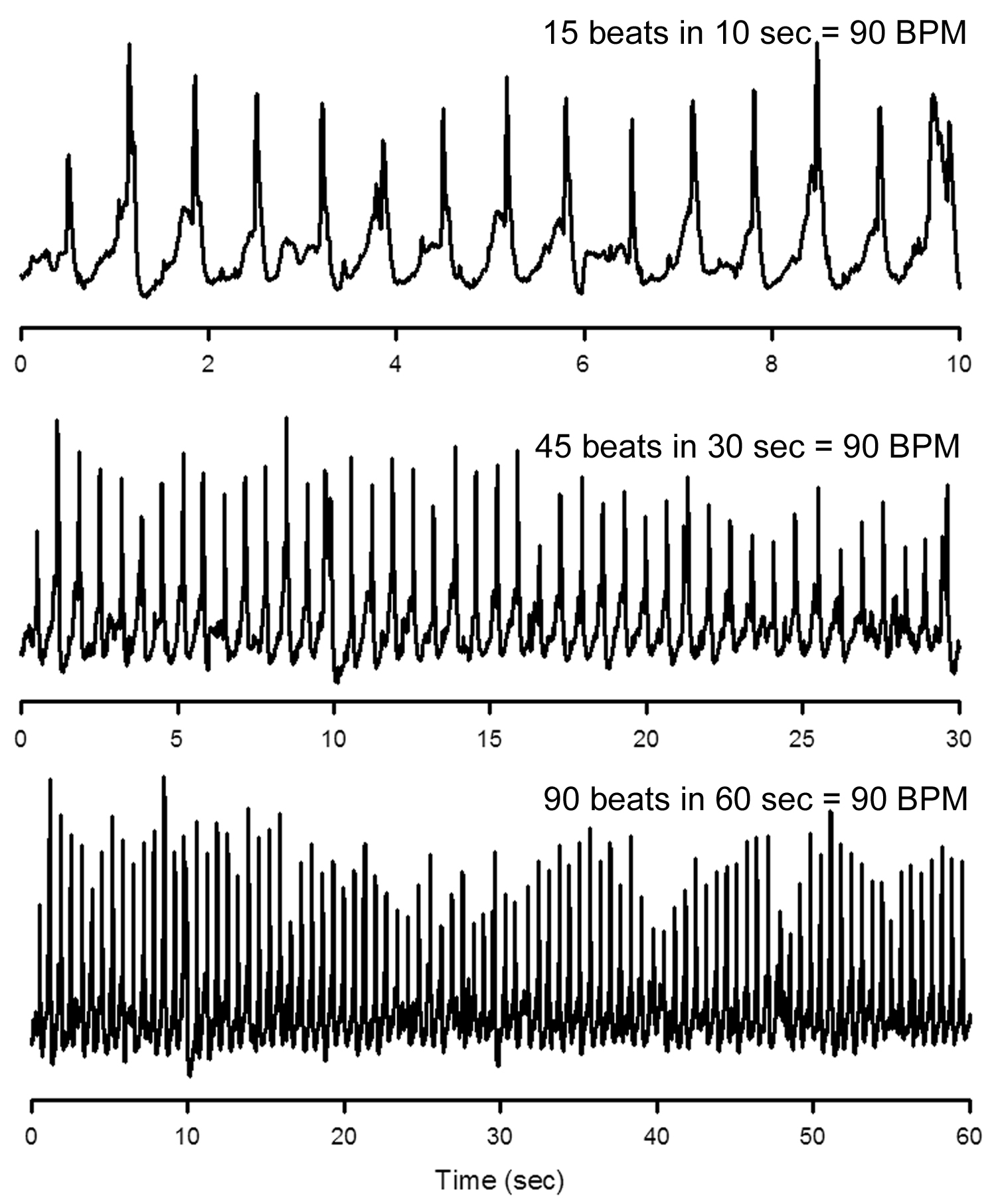 | |
 |  |  |  | |
 |  |  |  | |
 |  |  |  | |
 |  |  |  | |
 | ||||
| Back to TOP |