| (click on Topic) | |||
 | |||
| Integration of Sensory | |||
|
| |||
| Hairs | |||
| CRAYFISH Projects Related to Sensory Systems | |
| Back to General Crayfish Projects | |
| Back to General Research | |
| Back to Home Page | |
| |||||||||||||||||||||||
|
| |||||||
| Background
of SENSORY SYSTEMS related to our research interests The research goals related to sensory systems of my program are focused on understanding the physiological mechanisms of development and plasticity among sensory systems. Mine is a multifaceted approach from studying differences in ion channels on the surface of membranes to the neuromodulatory molecules whose actions on sensory systems are relevant to the whole animal. Recent research from a physiological perspective has demonstrated the mediating role of hormones, neural circuitry, and their interactions in generating individualistic and social behavior, though relatively little attention has been given to the key contributions of sensory systems and the autonomic nervous system. At a social level, animals show distinct differences in behavior and in responses to sensory cues. For example, among human siblings there may be dominant, outgoing individuals as well as shy and introverted ones; some individuals learn new tasks better and quicker than others. The focus of much research in this broad area has been on the central nervous system and has recently expanded into studies of the expression of particular genes in the nervous system, in a variety of animal species. The hope is to be able to get a handle on the mechanisms of how neurons are activated or turned off and how they communicate with each other to elicit particular responses. Such research in higher animals has proven to be a daunting task and many of the breakthroughs in neuroscience have arisen due to understanding of basic principles in simpler systems and then extrapolating to more highly evolved organisms, such as humans. The invertebrate arthropods have long provided key models, especially crayfish and Drosophila (T.H. Huxley, The Crayfish.1880; T.H. Morgan, 1900) for investigating neurophysiological principles. One advantage of invertebrates is that individual cells can be examined by a range of techniques from anatomical analysis to molecular genetics and electrophysiology, to obtain insights that are not possible, at present, in higher-animal model systems. In particular, the sensory neurons of crayfish and Drosophila serve as models to investigate the basics principles of neuronal function which is relevant to all neurons in all animals. Combining the physiological and behavioral perspectives in addressing these issues holds much promise for significant progress in the field. | |||||||
 | |||||||
| Altered states of excitability of sensory neurons : Proprioceptors | |||||||
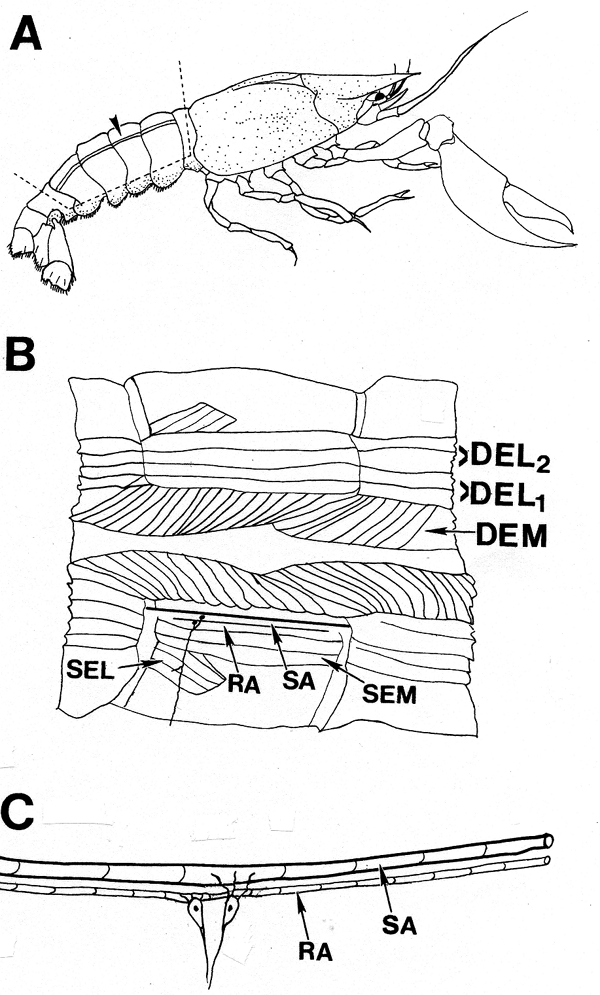
| The effects of invertebrate hormones serotonin (5-HT) and ecdysone (20-HE) on primary sensory neurons have only briefly been addressed in the literature. No reports to date have appeared on the effects of 20-HE in relation to non-genomic, rapid influences on invertebrate sensory systems. To determine the effects of 5-HT and 20-HE on a identified sensory cell neuron, we chose to use the abdominal proprioceptive sensory system of crayfish, referred to as the muscle receptor organs (MROs), which monitor the positions and movements of the abdominal segments (Eckert, 1961; McCarthy and MacMillan, 1995). This mechanoreceptor transduces the stimulus of stretch of the sensory endings into a receptor potential that is conducted past the cell body. If this potential exceeds a threshold, an action potential will result at the axon base. The neuron cell body is located in close proximity to the muscle it monitors. Two distinct types of stretch receptors exist in this sensory system--a slowly-adapting and a rapidly-adapting receptor. The activity pattern of the slowly-adapting MRO is dependent on the strength of the mechanical stretch. This MRO system in the crayfish is analogous to the intrafusal muscle spindle in mammals. However, the muscle spindle organs in vertebrates are challenging to investigate electrophysiologically, whereas the sensory neurons of the MRO are easily accessible to extracellular and intracellular electrodes for long term recordings. The cell bodies of the sensory neurons are relatively large (50-100 µm in diameter) and not contained in a ganglion as in vertebrates. The excised preparation also allows easy application and access of various neuromodulators to the neurons to examine potential effects. The natural flexion of the segments within the intact crayfish abdomen can be mimicked in an excised preparation examined in a dish containing a physiological saline. The MRO of the crayfish is a model sensory system that has been extensively studied for its basic properties since its description in lobsters by Alexandrowicz in 1951. This preparation has served well in understanding sensory transduction in general by the activation of ‘stretch activated’ channels (Erxleben, 1989). In addition, voltage-clamp and patch-clamp studies of the neuronal membrane have provided a fundamental understanding of ionic flow, channel distribution and density to regional properties of sensory neurons in general (Brown et al., 1978; Edwards et al., 1981; Erxleben, 1989; Hunt et al, 1978; Rydqvist and Purali, 1991; Rydqvist and Swerup, 1991; Purali and Rydqvist, 1992). From these past studies the localized region termed the ‘site of spike initiation’ was identified which related to a region of reduced threshold to activation. The early work of Eckert (1961) had illustrated that activation of the MRO in one abdominal segment can influence the activity of primary sensory neurons in an adjoining segment. This revealed processing within the segmental ganglion and influence of efferent control within the ventral nerve cord. We are examining both the chronic and the acute effects of 5-HT and 20-HE upon this primary sensory neuron by examining the changes in firing frequency in response to a static stretch, characteristic of the relatively inactive periods of movement observed for crayfish in their natural settings. We were also interested to know if the effects of the neuromodulators are dependent on the state of firing activity of the cell. These two points are fundamental to understanding sensory physiology and have not been well-addressed in mammalian or in invertebrate systems in respect to neuromodulation. | |||||||
|
| |||||||
 |
Altered states of excitability of sensory neurons: Cuticular sensory hairs
| ||||||
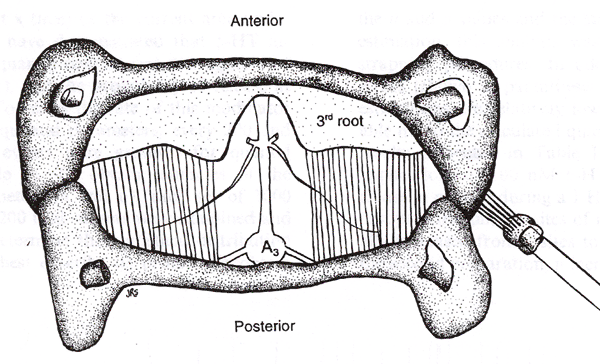
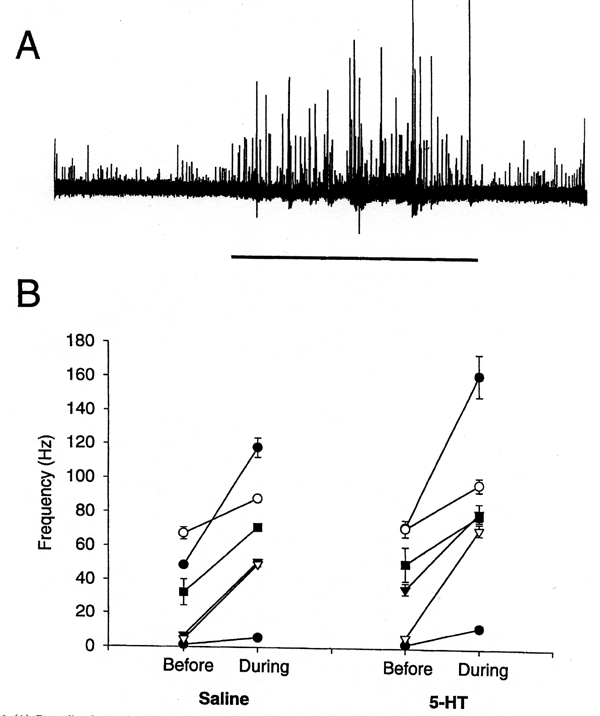
| This study illustrates the importance of determining the sensory contribution to the intrinsic activity of motor neurons, and demonstrates that 5-HT modulates not only the motor neurons but also sensory input to the pathway. This abdominal superficial flexor preparation in the crayfish allows one to address issues fundamental to all circuits and chemical synapses. Experiments were designed to assess the role of 5-HT in altering the activity of the sensory neurons which drive the motor neurons. Since the sensory axons are very small (1 to 10 µm) and difficult to monitor over time while changing the bathing medium, the entire distal 2nd root was monitored after it was transected from the ganglia. In the presence of 5-HT, there was an increase in the frequency of primary sensory neurons upon stimulation of the cuticle. This indicates that incoming information to the VNC is enhanced by 5-HT. For this paradigm, activity frequencies were quantified for one second immediately before the simulation and for the first second of stimulation in both saline and 5-HT. In three of the six preparations there was an enhancement of sensory neuron activity upon exposure to 5-HT without cuticular stimulation. From these studies we have shown that 5-HT even enhanced the sensory input that drives the flexor motor neurons (Strawn et al., 2000). | |||||||
 | |||||||
|
Altered states of excitability in integration of sensory information | |||||||
| The drive of the motor neurons can readily be examined by stimulation of various known sensory neurons, which enables one to assess their role in altering motor neuron activity. In addition, the integration of sensory information in the ventral nerve cord (VNC) which effects the motor output is accessible for study in the crayfish. Thus, the crustacean nervous system enables one to investigate each component of a sensory-ganglia-motor neuron-muscle pathway which elicits that behavior. In lobsters and crayfish, it has been shown that injections of serotonin (5-HT) into the circulatory system cause the animals to assume a semi-dominant posture (a raised extension of the chelipeds, although with a flexed state of their abdomen. Livingstone et al., 1980). The actions of 5-HT in eliciting these behaviors were shown to be selective within the VNC to enhance excitation of excitatory flexor motor neurons and decrease excitation of the inhibitor flexor motor neurons. Previous investigations did not address whether sensory neuron activity could be altered by 5-HT, and whether the alterations in sensory neuron activity could have an effect on the activity of the superficial motor neurons. It is well established in the Aplysia nervous system that 5-HT sensitizes a gill-withdrawal reflex (Siegelbaum et al., 1982) and it appears from the results presented herein in the crayfish system that 5-HT can also enhance sensory drive. The aims of this study were to examine the role of 5-HT on each component within a crayfish `sensory-ganglia-motor nerve-muscle' consisting of specific, identified cells within the second abdominal segment. This study illustrates the importance of determining the sensory contribution to the intrinsic activity of motor neurons, and demonstrates that 5-HT modulates not only the motor neurons but also sensory input to the pathway. We have shown that 5-HT enhances the motor drive in the presence of a given amount of sensory stimulation. Thus it appears that 5-HT alters the degree of integration of sensory input within the ventral nerve cord of the crayfish (Strawn et al., 2000). | |||||||
|
Aesthetasc sensilla on the antennules | |||||||
| Olfactory
sense | |||||||
|
A great photo from Dr. Thomas Breithaupt | |||||||
|
Neural activity is widely recognized as a contributing factor for appropriate neural development in a variety of animal species. The pioneering work of Wiesel and Hubel (1963) toward understanding the development of the visual system affected by the presence and absence of visual stimuli demonstrates how gross morphological alterations occur in synaptic organization in the various parts of the brain related to vision in cats. In a troglobitic (obligatory cave dwelling) crayfish species, it is known that these animals retain eye stalk musculature and reflexive eye movements such as: protective eye withdrawal, geotactic eye stabilization as well as proprioceptive nystagmus. The blind cave crayfish used in our study normally inhabit a cave environment containing water either as streams or pools in which no light is present. The blind cave crayfish, Orconectes australis packardi Rhoades have elongated antennae as compared to sighted crayfish. It is likely that this morphological characteristic of the crayfish is an adaptive trait for life in a cave. Past studies using the troglomorphic traits of elongated antennae and reduced eyes of the amphipod crustacean Gammarus minus have shown that these traits are genetically determined and that natural selection may be responsible for their evolution (Culver et al., 1995). Since the cave crayfish have a reduced visual system, it would not be surprising either tactile and/or chemosensory systems are enhanced. Detailed developmental studies in tracing olfactory afferent projections from the antennular nerve to the olfactory lobe, in the central brain, and interneurons projecting from the olfactory lobe to ganglia in the eye stalk have recently been undertaken (Schmidt, 1997; Harzsch et al., 1997). The olfactory projection neurons (OPN) have their cell bodies within the olfactory lobe and send their axonal projections, via the olfactory globular tract (OGT) to hemiellipsoid bodies in the eye stalk ganglion (Schmidt, 1997; Harzsch et al., 1997). The OGT forms a distinct sub-group of small axons within a region in the protocerebral tract. The protocerebral tract is the nerve bundle of the eye stalk that contains the axons to and from the ganglia within the eye stalk and the central brain, formally referred to as the optic nerve (Schmidt, 1997; Sandeman et al., 1998; Nunnemacher and Davis, 1966; Wiersma and Yamaguchi, 1966). The OPN bundle, has been identified in cross sections of the protocerebral tract within the shore crab, Carcinus maenas (Schmidt, 1997). We have already demonstrated that the cave crayfish have many more second order neurons dedicated to chemosensory information as compared to epigean (surface) crayfish (Cooper et al., 2001), but more studies are needed for a broader comparative investigation. Our purpose is to compare morphological differences of the chemosensory structures of surface and troglobitic crayfish. We have started counting the number of aesthetasc sensilla on the antennules of various crayfish and have very encouraging results to date. There are also a number of crayfish species across the state of KY that are housed in a collection museum at EKU under the directorship of Dr. Schuster. Dr. Schuster is a world authority on crayfish life history and their biology. This work will be done in collaboration with Dr. Guenter Schuster at EKU. This research topic raises an issue of environmental concern on water quality, particularly for cave crayfish which do not have an option to make use of visual cues. What impact would chemical pollutants have in the ability of the animals to detect important pheromone signals for reproduction ? Or maybe even predator detection? These questions are now being tackled using epigean (surface dwelling) crayfish in order to postulate the potential threats to cave crayfish as well as other cave-adapted organisms. With the recent developmental plans of airports, truck depots and highways over extremely biological and geological fragile areas such as in the karst plans of western Kentucky (in the vicinity of Mammoth cave) the effects of toxic chemical will unfortunately directly test their impact on the survival of many cave-dwelling and troglobitic species. We will be working to continually survey the impact on the cave ecosystems in relation to altered olfactory communication. | |||||||
 | |||||||
| Whole animal perception | |||||||
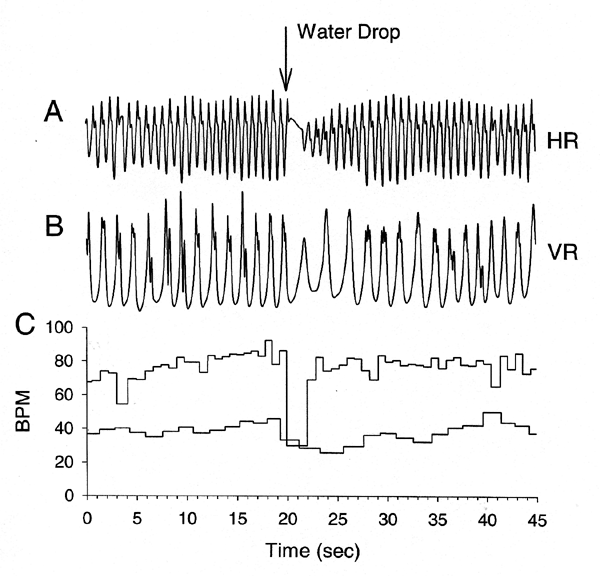
| As with all crustaceans investigated, crayfish are very much aware of their surroundings and react to even the slightest of disturbances. No where is this more apparent in a still, shallow pool of water within a cave in which the slightest vibration will set off ripples traveling through the water which will quickly be monitored by the elongated antenna of a crayfish. What will determine if the crayfish flees or waits ? Is it the strength of the stimulus or interval at which they occur? Or maybe differences in an individuals physiological state will determine if the animal will flee or fight it out. These are the types of questions that we are tackling to answer. In order to better understand what external and internal factors influence the animals behavior one has to know the types of senses the animal possess and the range in sensitivity of these senses. One might casually think that since a particularly troglobitic crayfish does not contain pigment in their eye caps or even rudimentary sensory structures in the eye that they are not sensitive to light, however this is a misconception. The “blind cave crayfish” are responsive to light by a photo receptor that is within their nerve cord contained in the tail. They can not see with this photo receptor but they can detect if white light is shining on them. We recently have demonstrated that white light does alter the types social interactions that normally occur with cave crayfish (Li and Cooper, 2002). We have also shown that red light or IR light does not cause any physiological changes in heart rate (HR) and ventilatory rates (VR), however exposure to white light dramatically alters both HR and VR (Li et al., 2001). In addition we have shown that the duration of social contact is decreases among two crayfish when they encounter each other in the presence of white light. Thus, long term consequences might even alter reproductive success and ultimately the population of the species. What is so nice by monitoring HR and VR in the relatively rare cave crayfish is that it is non-life threatening. Two little sterile wires are placed just under the cuticle on the back of the crayfish and when the recording is over the wires can either be removed or just cut off close to the cuticle so that when the animal molts the ends of the wires are shed along with the old exoskeleton. Since working in the field within the various caves has proven to be difficult, we remove crayfish from the cave environments to retain them in a cave-like environment within our laboratory at the University of Kentucky. This serves many useful purposes since within defined environmental conditions we can exclude various environmental cues such as olfactory signals which alter how the crayfish respond to various stimuli. In addition, we can add specific cues and examine their influence on individual and social behavior. These studies had demonstrated that HR & VR represents a functional state that relate to environmental changes, thus allowing one to index an autonomic state of the animal (Listerman et al., 2000; Li et al., 2000; Schapker et al., 2001). The responses in crustaceans to environmental disturbances are similar to those of vertebrates that are regulated by autonomic control. The purpose of our study is to determine if simultaneous measures of HR and VR may serve as good biological indices to use in crayfish to assess if an animal is sensitive to alterations in its environment during which time behavioral observation would not be as favorable for assessment. In addition, we wanted to know if measures of HR and VR would provide insight into the function of the autonomic state of crayfish prior and during agonistic encounters. | |||||||
 | |||||||
| Influence of animals intrinsic state | |||||||
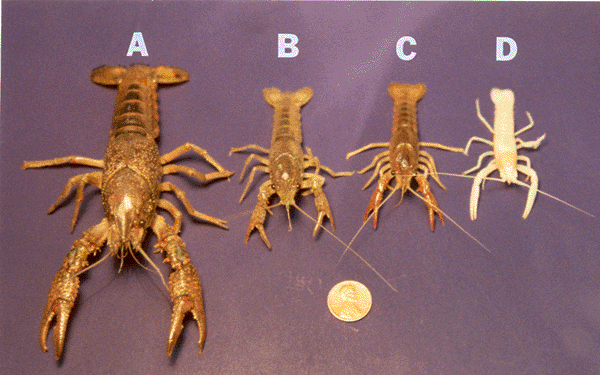 | |||||||
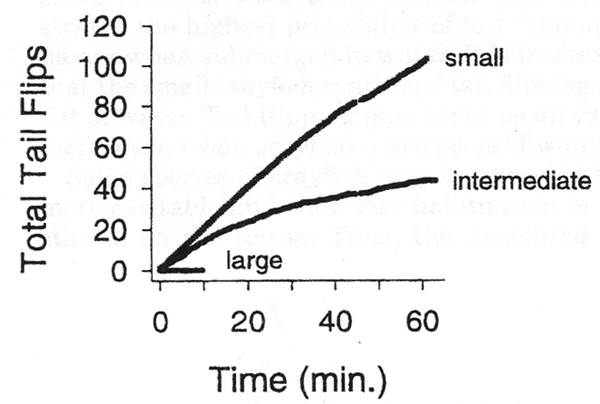
| Prey use several tactics in order to evade predators. These include hiding, being motionless, or rapidly escaping. For instance, crayfish have developed the tail flip to escape the predation of birds, fish, reptiles, and other crayfish. Different responses are utilized for different threats and these responses may change as the animal grows. How animals respond to various sensory stimuli during different developmental stages of their life depends on many factors. For example, smaller crayfish are more likely to tail flip during an encounter with a larger crayfish (Pavey and Fielder, 1996), and experience plays a role in determining this behavior (Copp, 1986). Also, responses to various stimuli depend on the sensing acuity of an animal. Much of our understanding of sensory systems is derived from examining individual sensory systems isolated from the organism as a whole (Atema et al., 1988). Approaching the situation in this manner does not accomplish the ultimate goal of understanding the animal’s behavior (Atema et al., 1988; Burmistrov and Shuranova, 1996; Zulandt-Schneider et al., 1999). The evolutionary changes observed in blind cave crayfish suggest the importance of nervous system plasticity in promoting the survival of the species. In addition to providing a good comparison for blind cave crayfish, sighted crayfish make a good model for further exploring the sensory system involved in the tail flip response. In most animals, the dissection of individual neurons and neural circuits to understand the complex integration of sensory and motor systems is extremely challenging. Crayfish serve well as a model organism for investigating escape behaviors. They have evolved particular behaviors that can be quantified and investigated even at the level of neural circuitry. For example, the tail flip response in relation to the age of the animal and the size of the neurons with the circuit have been examined (Fricke, ‘86). Furthermore, the sensory difference in a blind cave crayfish (Orconectes australis packardi) suggests that they might have evolved a different responsiveness to tailfliping in relation to the developmental stages. It seems reasonable that the blind cave crayfish do not have the advantage of responding to visual stimuli through various neuronal circuits to elicit a tailflip as due sighted crayfish. On the other hand, it is assumed that the blind cave crayfish rely heavily on their other senses to meet the challenges of cave life in total darkness (Barr and Holsinger, ‘85; Culver et al., ‘95). These unique adaptations may show differences in the habituation of the crayfish due to changes in the animal’s sensory state. Comparing the blind cave crayfish responses with previous studies on sighted crayfish provides insight into the factors that drive selection for the tailflipping behavior. Previous studies dealt with the tail flip response being advantageous to the animal at various stages of its life. Juvenile, sighted crayfish are more likely to respond to threatening stimuli by tailflipping, while adult crayfish are more likely to respond with the defensive use of their chelipeds. Fricke (1986) noted that the chelipeds of the juvenile crayfish account for little of the animal’s total body mass when compared to that of an adult. Thus, juveniles can tail flip without the energy cost of moving the large mass of the cheliped. Morphologically, the thinner exoskeleton of small crayfish makes them less able to withstand the stress of predation. Consequently, they may have evolved greater tailflipping capabilities. Mature adults of Procambarus clarkii will not exhibit tailflips when both of their chelipeds are intact, and it has been suggested that the disproportionate development of cheliped mass to body mass of the animals does not favor tail flipping in adults (Fricke, 1986). We have shown that O. a. packardi exhibit even greater ration of cheliped length to body length. Yet, a much higher occurrence of tail flipping exists in adult cave crayfish than for adults of P. clarkii. Conclusively, tail flipping behavior differences among species of crayfish is not just a function of body proportions. The blind cave crayfish’s sensory state may be an additional factor in generating tail flipping behavior. Another facet, the neural circuitry of the tailflip response, has been examined extensively (Krasne and Wine,1975; Olsen et al., 1996; Yeh et al., 1997; Krasne et al., 1997). Krasne and Wine (1975) identified three pathways crayfish use to elicit a tailflip response: lateral giant, medial giant, and non-giant. Krasne and Wine (1975) used touches on the tail in order to elicit lateral giant responses. They initiated further studies of the neural circuitry by eliciting lateral giant responses to drive the tail flip behavior. Subsequently, Fricke (1986) noted that stimuli given every 30 seconds caused intermediate sized (>10 cm) crayfish to show habituation. The same protocol used on smaller individuals elicited habituation resistant behavior. Later, a physiological explanation was proposed by Edwards et al. (1994). As a crayfish grows, there is an increasing predominance of the depression-prone synapses in carrying information from the sensory neurons. These results demonstrate that larger animals have a faster rate of habituation than do smaller ones. A link has been established between claw removal and the tendency to increase tailflips generated by medial and non-giant pathways (Krasne and Wine, 1975; Lang et al., 1977). These previous studies have examined modulation of the crayfish tail flip response over long periods of time; however, they have not examined the habituation of the tailflip response for possible modulatory influences over a short term basis (i.e., seconds). It seems reasonable that the survival of the crayfish would be aided by the ability to modulate the tail flip response. Change within short periods of time and to a variety of factors would promote crayfish survival. The tailflip response could be modulated from direct neural input within the circuitry and/or by release of neuromodulators form ‘gain setting’ neurons. To examine habituation rates in the tail flip response based on size of P. clarkii, three distinct size groups were tested. The group of large individuals consisted of the largest adults commonly found in the field (Raceland, LA). The size of small crayfish compares to earlier reports on habituation of tail flip response (Fricke, ‘86). Small crayfish were the same size as the adult cave crayfish used in this study. The intermediate-sized group of P. clarkii provided a third grouping to determine a size relationship in the habituation response. This study demonstrates that the probability of the crayfish, P. clarkii, to tailflip in response to a touch on the dorsal tail fan is dependent on the size of the animal and the behavioral state of the animal (Kellie et al., 2001). The larger the animal, the less likely it is to tailflip. Also, a size dependency for an increased tendency for exhibiting habituation to the stimuli with repetitive trials exists. Altering the animal’s physical state by autotomizing the chelipeds increases the tailflip response in larger animals. Altering the environment, such as to one with little water depth, also can cause crayfish to respond differently to the stimulus. For example, small crayfish will habituate more rapidly when placed in shallow water. Observations of adult crayfish of a species adapted to live in cave darkness revealed that they are more likely to tailflip than sighted adults of a different species. When the troglobitic crayfish were examined within a group, they exhibited a decrease in general movement over time. Thus, interaction instances decreased. However, the probability to tailflip remained constant in spite of the decrease in interactions. | |||||||
|
| |||||||
| Listerman,
L., Deskins, J., Bradacs, H., and Cooper, R.L. (2000) Measures of heart rate during
social interactions in crayfish and effects of 5-HT. Comparative Biochemistry
and Physiology A.125:251-264 Li,
H., Listerman, L., Doshi, D., and Cooper, R.L. (2000) Use of heart rate to measure
intrinsic state of blind cave crayfish during social interactions. Comparative
Biochemistry and Physiology A.127:55-70. Strawn,
J.R., Neckameyer, W.S., and Cooper, R.L. (2000) The effects of 5-HT on sensory
neurons, CNS command, and neuromuscular junctions of the crayfish abdominal superficial
flexor. Comparative Biochemistry and Physiology B 127:533-550. Cooper,
R.L., Li, H., Long, L.Y., Cole, J., and Hopper, H.L. (2001) Anatomical comparisons
of neural systems in sighted epigean & troglobitic crayfish species Journal
of Crustacean Biology 21:360-374. Li, H. and Cooper, R.L. (2001) Spatial familiarity in the blind cave crayfish, Orconectes australis packardi. Crustaceana 74: 417-433. (Li was my PhD student). Kellie,
S., Greer, J. and Cooper, R.L. (2001) Alterations in habituation of the tail flip
response in epigean and troglobitic crayfish. Journal of Experimental Zoology
290:163-176. Li, H. and Cooper, R.L. (2002) The effect of ambient light on blind cave crayfish: Social interactions. Journal of Crustacean Biology 22:449-458 (Li was my PhD student). Schapker,
H., Breithaupt, T., Shuranova, Z., Burmistrov, Y. and Cooper, R.L. (2002) Heart
rate and ventilatory correlative measures in crayfish during environmental disturbances
& social interactions. Comparative Biochemistry and Physiology A 131:397-407.
[FullText.pdf]. Cooper, R.L., Ward, E., Braxton, R., Li, H., and Warren, W.M. (2003) The effects of serotonin and ecdysone on primary sensory neurons in crayfish. Microscopy Research and Technique 60: 336-345. [PDF] (Ward, Braxton, and Warren were undergraduates and Li was a graduate student in my laboratory). Ziemba, R., Simpson, A., Hopper, R., and Cooper, R.L. (2003) Comparisons of aesthetasc sensilla on the lateral antennules between two epigean (surface) species and the blind cave crayfish Orconectes australis packardi. Crustaceana 76: 859-869. [PDF] (Anna Simpson was an undergraduate in my laboratory at UK). Shuranova, Z.P., Burmistrov, Y.M., and Cooper, R.L. (2003). A hundred years ago and now: A short essay on the study of the crustacean hindgut. (Vor hundert Jahren und nun: Eine kurze Geschichte von die Forschung des Hinterdarmes der Crustaceen). Crustaceana 76:755-670 [PDF]. Shuranova, Z.P., Burmistrov, Y.M., and Cooper, R.L. (2003). Activity of the ventilatory muscles in the crayfish. Comparative Biochemistry and Physiology A. 134: 461-469 [PDF] (Drs. Shuranova and Burmistrov are a Russian husband and wife team and are collaborators). Tilden, A.R., Brauch, R., Ball, R., Janze, A.M., Ghaffari, A.H., Sweeney, K., Yurek, J.C. and Cooper, R.L. (2003) Modulatory effects of melatonin on behavior, hemolymph metabolites, and neurotransmitter release in crayfish. Brain Research 992:252-262 [PDF] Pagé, M.-P. and Cooper, R.L. (2004) Novelty stress and reproductive state alters responsiveness to sensory stimuli and 5-HT neuromodulation. Comp. Biochem. Physiol. A 139:149-158 [PDF]. Shuranova, Z.P., Burmistrov, Y.M., Strawn, J.R., and Cooper, R.L. (2006). Evidence for an Autonomic Nervous System in Decapod Crustaceans. International Journal of Zoological Research 2(3):242-283. [Full text PDF] color figures. | |||||||
| Back to General Research | Back to the TOP | |
| Back to Home Page |