| FUTURE RESEARCH | |||
| Back to General Research | |||
| Back to Home Page |
|
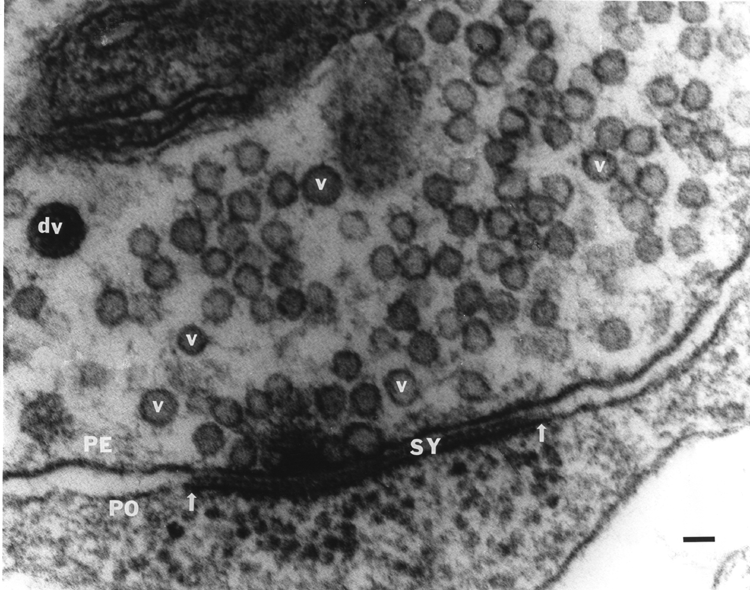
Through the use of recently developed proteins and fluorescent dyes, we have begun to investigate mechanistic questions of neurotransmitter release. Intracellular injection of vesicular docking proteins into the large axons of crustacean motor neurons in the absence or presence of neuromodulators allows one to investigate if the intracellular signaling systems involved work independently or synergistically to affect synaptic efficacy and ultrastructure. The crayfish leg opener muscle has provided a great deal of insight into the basic mechanisms of synaptic transmission because it allows neurotransmitter release to be directly related to synaptic physiology and structure (Atwood et al., 1994; Atwood and Cooper, 1995, 1996a, b; Cooper et al., 1995a,b; 1996a,b). In this preparation, the relatively low output of each varicosity along the nerve terminals allows the use quantal analysis and statistical evaluation of individual vesicular release events. The initial investigations of a synaptically-relevant molecule, alpha-SNAP (He et al., 1999; MS Thesis from my lab) suggest that other molecules may be used similarly to address their functional significance in synaptic transmission. Molecules that dock synaptic vesicles are of special interest. There is particular current interest in molecules like 5-HT and octopamine (OA) (and their attendant signaling cascades) because of their ability to alter synaptic transmission. When applied singly to the NMJ, 5-HT and OA enhanced transmission, but if 5-HT is applied after OA, synaptic efficacy is reduced. Since these compounds co-exist in the hemolymph, I decided to investigate their effects at the neuromuscular junction following sequential and combined exposure in order to better understand their actions within the animal. To my surprise, we found that in some preparations, OA showed the well known enhancement of transmitter release, but there was also a number of junctions that showed reduced transmitter output. This is in accord with a recent finding that OA also reduces transmitter release at larval Drosophila neuromuscular junctions (Nishikawa and Kikodoro 1999), although for other insect preparations an increase of EJP amplitudes by OA is reported (for review see Roeder, 1999). In crayfish and crab NMJs, 5-HT always potentiated transmitter release and this effect was invariably reduced in the presence of OA, suggesting that when the two amines are present together they antagonistically modulate transmitter release. We address, with quantal analysis, the presynaptic actions of 5-HT, OA and a mixture of the two neuromodulators to begin to understand the underlying mechanisms of action. We currently have a manuscript with Dr. Rathmayer (Univ. of Konstanz, Germany) on this matter. It is thus likely, considering our recent physiological results that activation of the cAMP mediated processes from initial OA stimulation can dampen the subsequent IP3 mediated responses induce by the rapid actions of 5-HT. The long term action of 5-HT mediated through cAMP (Dixon and Atwood, 1989) may also be reduced if the same downstream actions are already activated by the prior presence of OA and a complete recovery has not been allowed to occur. Much work remains to determine which proteins and/or additional cytoplasmic messenger systems may be recruited into or out of action in the presence of just 5-HT or OA alone. This may then lead to an understanding of the antagonist actions of OA to subsequent 5-HT applications as we have reported. Besides 5-HT and OA there are numerous other neuromodulators working in concert that influence synaptic function and which ultimately may also help to regulate the behavior of the whole animal. In the future, I intend to address the interactions of various cascades with calcium regulation inside nerve terminals and their relation to neural activity. Several other projects will be pursued simultaneously in the future related to developing novel methods to assess synaptic transmission. Past methods of quantal analysis that I have developed have been well-received by the scientific community. At present I am developing novel statistical methods to describe fluctuation in synaptic responses. These responses are examined with the concept in mind that structural elements of the synapse may be the basis for differences in fluctuation among the various types of synapses. In order to develop this line of study, I needed to further establish new ways to more accurately quantify synaptic structures observed in electron micrographs. These studies have recently been published in Kim et al., 2000 and Feurverger et al., 2000. It is very important to understand, quantitatively, the scale of morphological features in 3-D when viewing the morphology in 2-D in order to draw comparisons between specimens. This is especially relevant to my projects of ultrastructural differences in high- and low-output synapses and interconversion of phasic to tonic motor neurons as well as developmental issues of the neuromuscular junctions.
|
| Multiple research disciplines are being examined separately but they are all interrelated and focus on the issue of synaptic function. With the successful outcome of published findings over the last 4 years from my research program, I believe this approach will continue to be productive. |  |